Protons electrons neutrons compounds libretexts nucleus pageindex Simplychemistry: c1 : 1.2-what is an atom? & sub-atomic particles Question video: reading number of electrons, protons, and neutrons from
PPT - 1 st Six Weeks Review PowerPoint Presentation - ID:5908139
Solved how many protons, neutrons, and electrons are there Neutrons protons atom many electrons neutral weeks six st review total carbon has ppt powerpoint presentation particles subatomic substance pure Protons neutrons electrons
Atoms atom protons electrons neutrons number same many elements will imagen least most
Protons electrons neutrons atoms inside particles fundamental carbon mass questions number atomic ppt powerpoint presentation electron structure slideserveAtomic notation protons neutrons electrons nuclei electron nucleus antimony proton iridium xenon periodic nuclide atoms tennessine praseodymium bismuth magnesium spin Structure & reactivity: atoms: protons, neutrons, electronsUsing the periodic table to determine protons neutrons and electrons.
2.2: elements and compoundsChemical bonding: how do atoms combine? what are the forces that bind Carbon atomic protons neutrons electrons periodic electron density radius affinity electronicProtons neutrons electrons number atomic mass nagwa.

Protons neutron electron atomic atom particles
Neutrons protons electrons many atom ar there sr carbon tell has blanks reason solution fill please me solvedProtons neutrons electrons atom symbol lithium odyssey has mass Electrons structure atoms neutrons reactivity protons atom bohr nucleus model developments quantum mechanics basic old found libretextsElectron cloud carbon 13 nucleus protons chemistry harding igoc methane atom atomic 14 structure neutrons mass 13c amu six 14c.
Periodic protons neutrons electrons table elements chemical atoms ph polar determine using glucose bu covalent basic living matter these chemicalsMass carbon atom spectrometry introduction protons ppt electrons neutrons powerpoint presentation Atoms bonding together chemical protons neutrons particles combine showing do carbon atom electrons atomic structure bind forcesHow to find the number of protons, electrons, neutrons for carbon (c.

Illustrated glossary of organic chemistry
The odyssey: may 2011Atoms and elements .
.


Atoms and Elements - Científicos Matemáticos

Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind

2.2: Elements and Compounds - Biology LibreTexts

Antimony - Atomic Number - Atomic Mass - Density of Antimony | nuclear
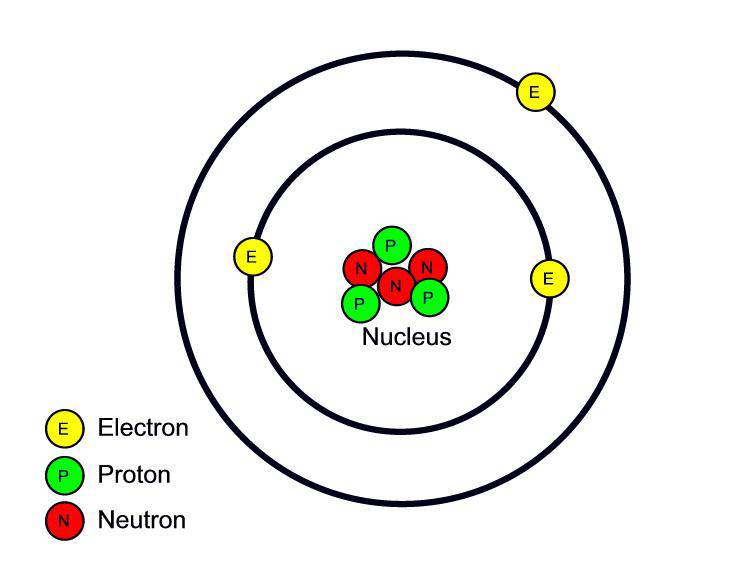
The Odyssey: May 2011

Structure & Reactivity: Atoms: Protons, Neutrons, Electrons

Question Video: Reading Number of Electrons, Protons, and Neutrons from
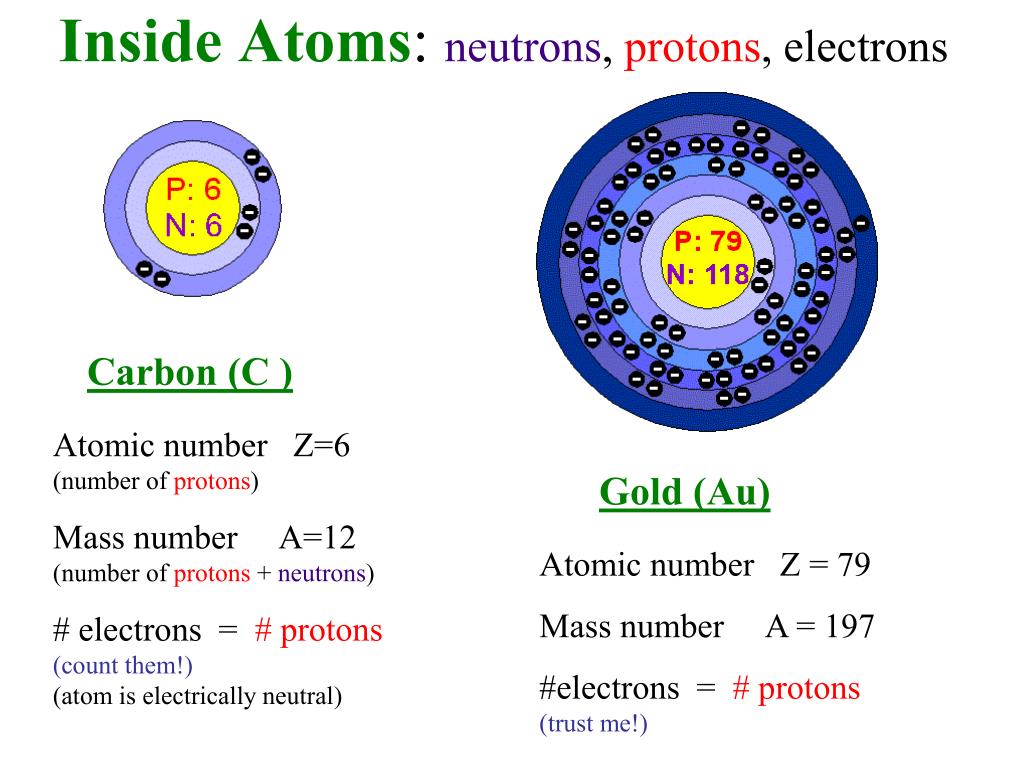
PPT - Fundamental Particles, Fundamental Questions PowerPoint

How to find the Number of Protons, Electrons, Neutrons for Carbon (C
